Insights

TCT 2023 Highlights: CathWorks Leaves its Mark
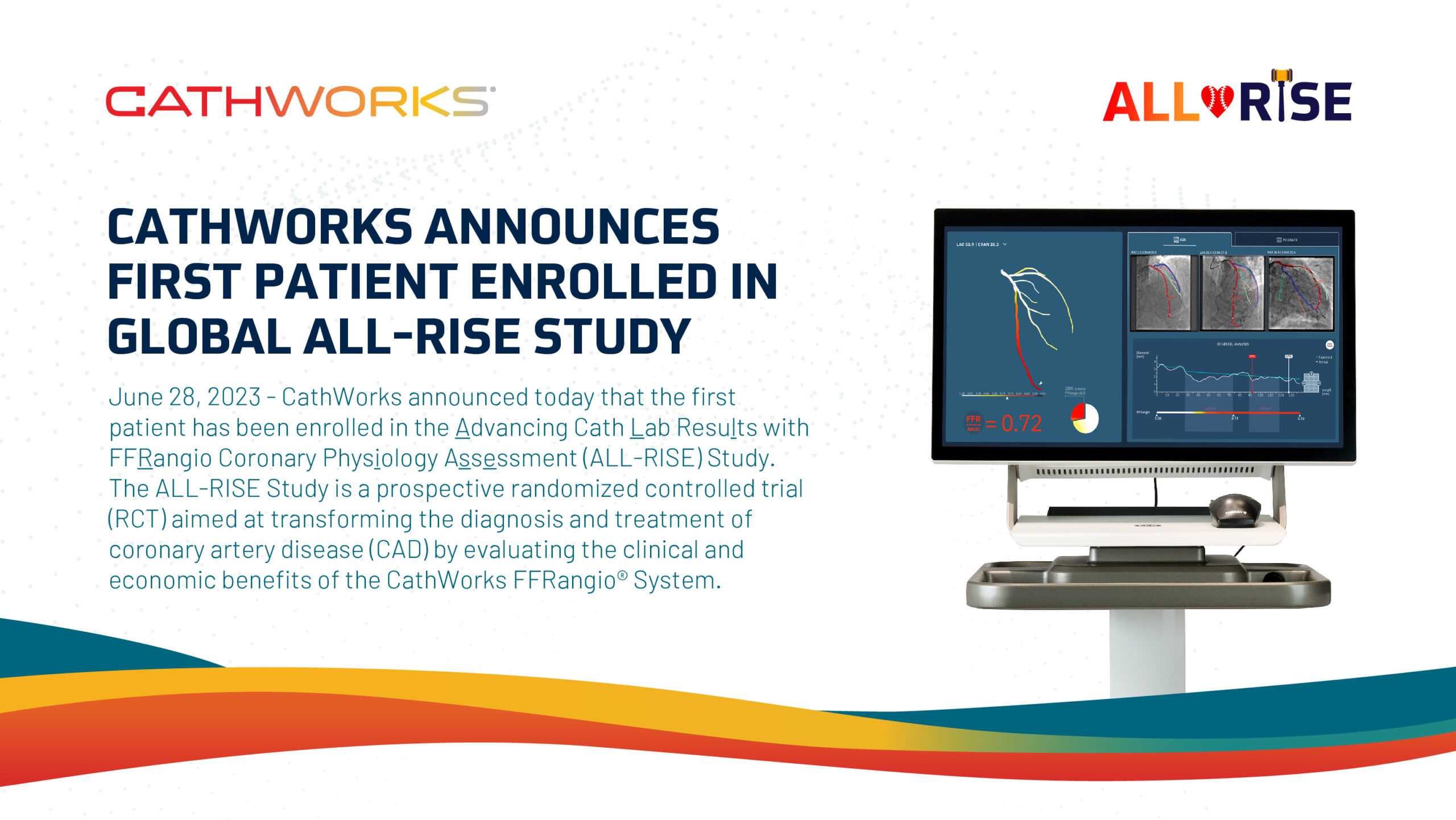
The CathWorks FFRangio® System generated a lot of interest and excitement from interventional cardiologists at the annual TCT conference in San Francisco.…
Get the latest CathWorks news, press releases, industry insights, and useful information for physicians, patients, and media.