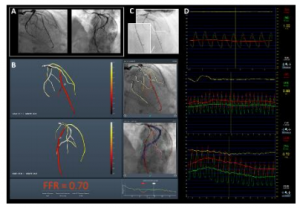
KFAR-SABA, ISRAEL (September 24, 2018) — The journal Circulation published results from the CathWorks FFRangio™ FAST-FFR clinical trial in an article titled Accuracy of Fractional Flow Reserve Derived From Coronary Angiography. The publication and trial results were announced today at the 2018 TCT (Transcatheter Cardiovascular Therapeutics) annual meeting in San Diego, California.
The FAST-FFR trial demonstrated that the sensitivity and specificity of the CathWorks FFRangio System were 93.5% and 91.2%, respectively, both of which exceeded the trial’s pre-specified performance goals. The diagnostic accuracy was 92% overall, and remained high when considering only FFR values in the critical zone between 0.75 and 0.85. As a result, the trial concluded that the CathWorks System has the promise to substantially increase physiologic coronary lesion assessment in the cath lab, potentially leading to improved patient outcomes.
The CathWorks FFRangio System is a non-invasive FFR platform that quickly and precisely delivers objective multi-vessel physiologic measurements to cost-effectively optimize and confirm intraprocedural percutaneous coronary intervention (PCI) therapy decisions. It is designed to deliver the objective FFR guidance needed to optimize PCI therapy decisions for every patient.
Stephan Achenbach, MD, FSCCT is Chairman of Cardiology and Professor of Medicine at the University of Erlangen, Germany, and was the leading enroller in the FAST-FFR trial. Dr. Achenbach explained the importance of the clinical findings in this trial; “Guidelines mandate that revascularization decisions are based on the presence of ischemia. However, not every stenosis or invasive angiography, even if perceived as ‘severe,’ causes ischemia. Equally important, lesions that do not appear severely stenotic may cause ischemia at times and may hence benefit from revascularization. Invasive angiography in such cases is incomplete without assessment of ischemia. Invasive FFR is considered the standard of care in these situations but adds cost and a significantly higher level of invasiveness to the angiogram, leading to pronounced under-use in clinical reality. Enrolling many patients in the FAST-FFR trial, my team and I experienced how easily the CathWorks FFRangio System can be added to the angiogram. The published trial results convincingly demonstrate the clinical benefit that may be derived. The CathWorks System may turn out to be disruptive technology that changes the workflow in the cath lab – to the benefit of our patients.”
The FAST-FFR trial was rigorous. CathWorks FFRangio System data was measured by 19 on-site cath-lab clinicians blinded to the invasive FFR measurements. Angiograms were acquired by dozens of operators at ten hospitals using all four of the major angiography systems (Siemens, Phillips, Canon, and GE). In addition, a majority of subjects were overweight or obese, and 20 percent had calcified lesions. In the presence of all of these real-world conditions, CathWorks FFRangio accuracy was still very high.
William Fearon, MD, Professor of Cardiology at Stanford Medical Center in Palo Alto, California, was the Principal Investigator of the trial and lead author. Dr. Fearon pointed out that “The results of the FASTNEWS RELEASE Contact: Jim Corbett, CEO +1 949.395.1214 [email protected] Rapaport Street Kfar-Saba 4465141, Israel | cath.works FFR trial demonstrate a very high accuracy and strong correlation between the reference standard, pressure wire-derived FFR and FFRangio. Based on these findings, FFRangio has the potential to substantially increase physiologic coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes.”
The CathWorks FFRangio System is in development and is not yet FDA- cleared.