CathWorks FFRangio® System Clinical Evidence
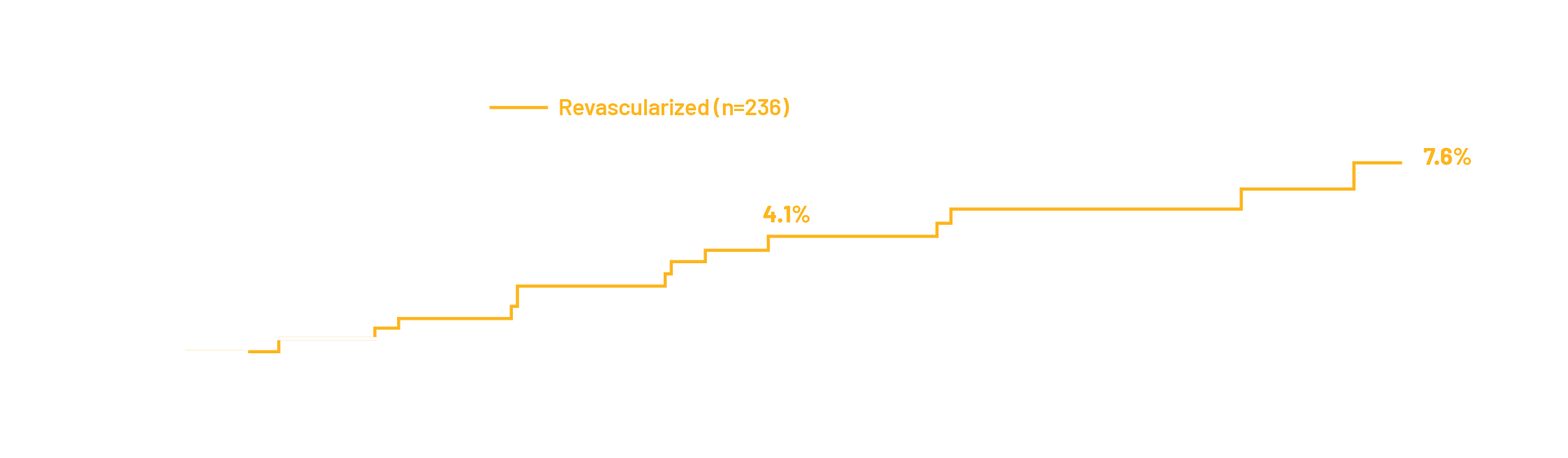
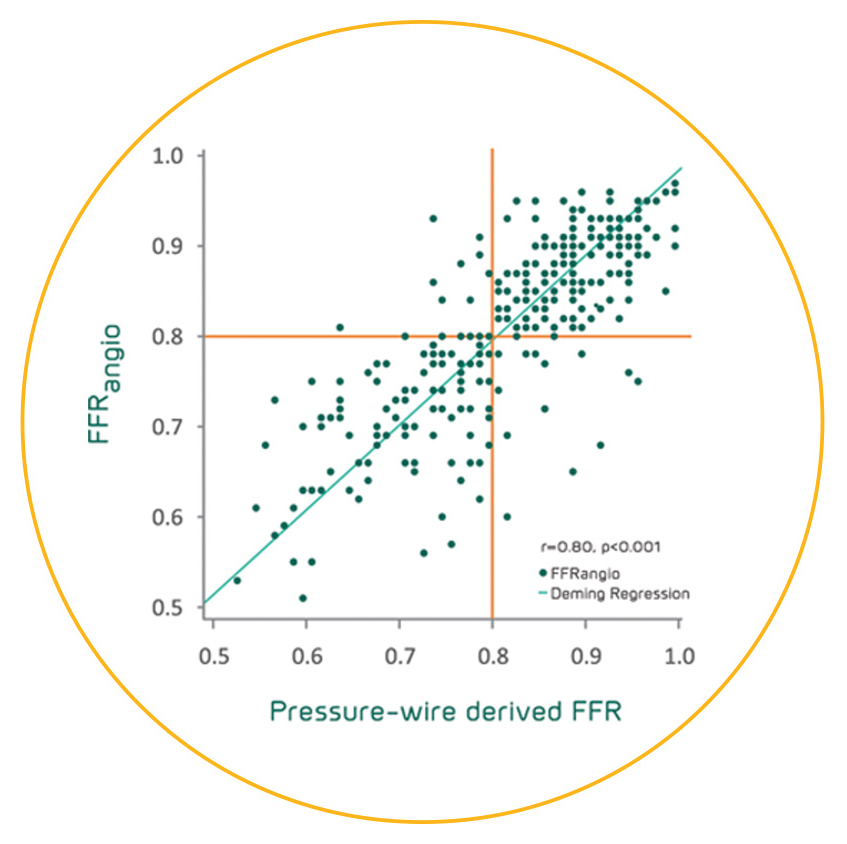
The FFRangio System demonstrates excellent diagnostic performance compared to invasive wire-based FFR and promising outcomes out to 2 years for both revascularized and deferred patients when treated according to FFRangio System guidance.